SaMD Regulations Wizard
SBRI Development funded Innovation
Overview
Funded by SBRI Healthcare, this project delivered a proof-of-concept platform that helps digital health product owners navigate medical device regulations, from classification to technical file preparation.
The result was The Regulations Wizard, an interactive prototype designed to simplify and de-risk regulatory compliance for Software as a Medical Device (SaMD) — using plain language, tailored checklists, and expert-backed workflows.
My contribution
- Product design & prototyping
- User research & wireframing
The team
- CTO
- 1x Product designers
- 2x Product managers
- Director of research
- Research associate
- Regulation experts
Year
2021-22

Product brief
The goal was to create a scalable, intelligent tool that empowers digital health innovators to confidently navigate medical device regulations, reducing reliance on costly consultants, cutting time to market, and improving compliance outcomes. By combining expert-driven logic with user-centred design, we aimed to bring clarity, speed, and structure to one of the most complex barriers in digital health innovation.
Key deliverables
-
A fully clickable, user-tested prototype built in InVision, showcasing the end-to-end user journey from classification to documentation, designed for both first-time and experienced product owners.
-
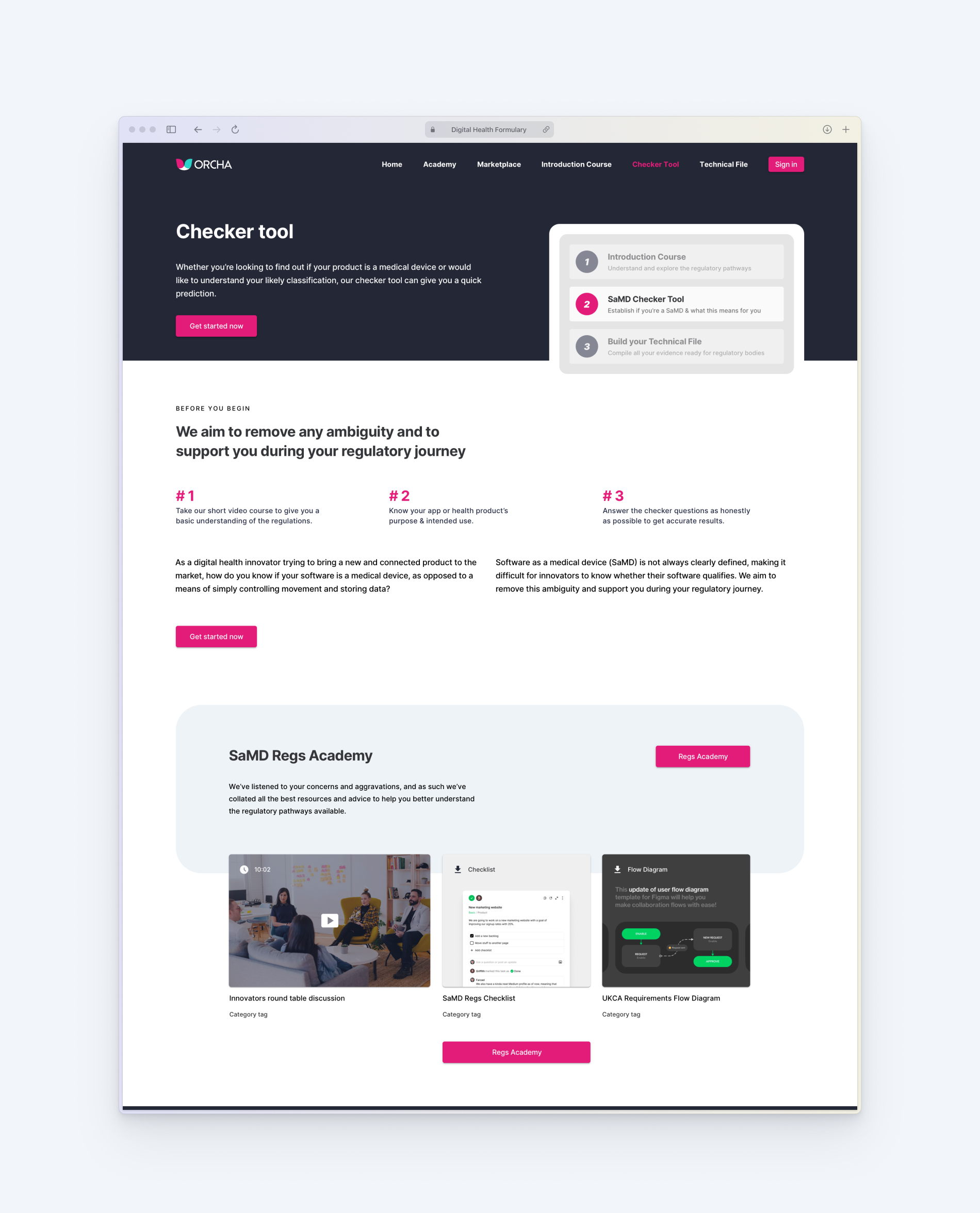
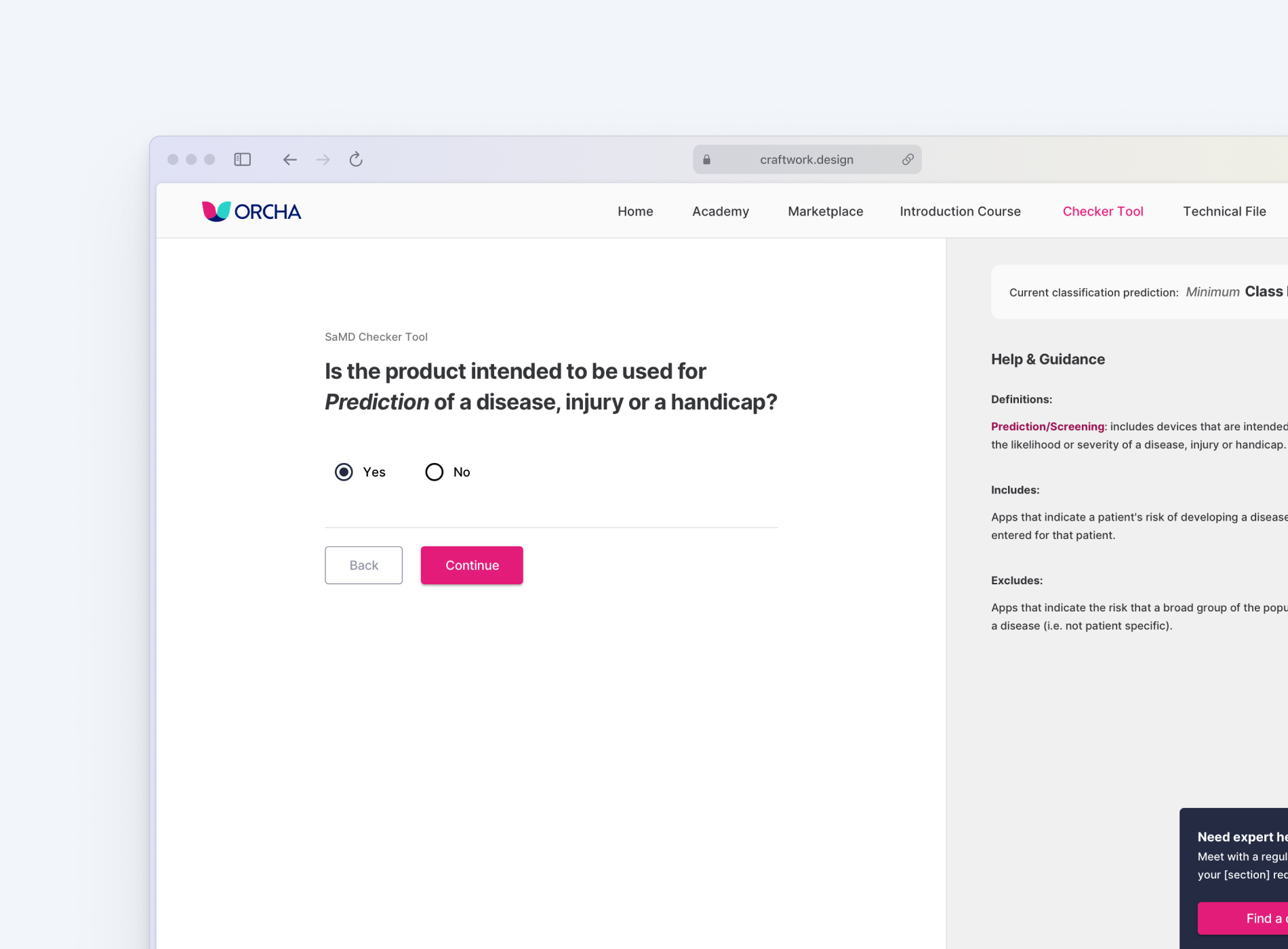
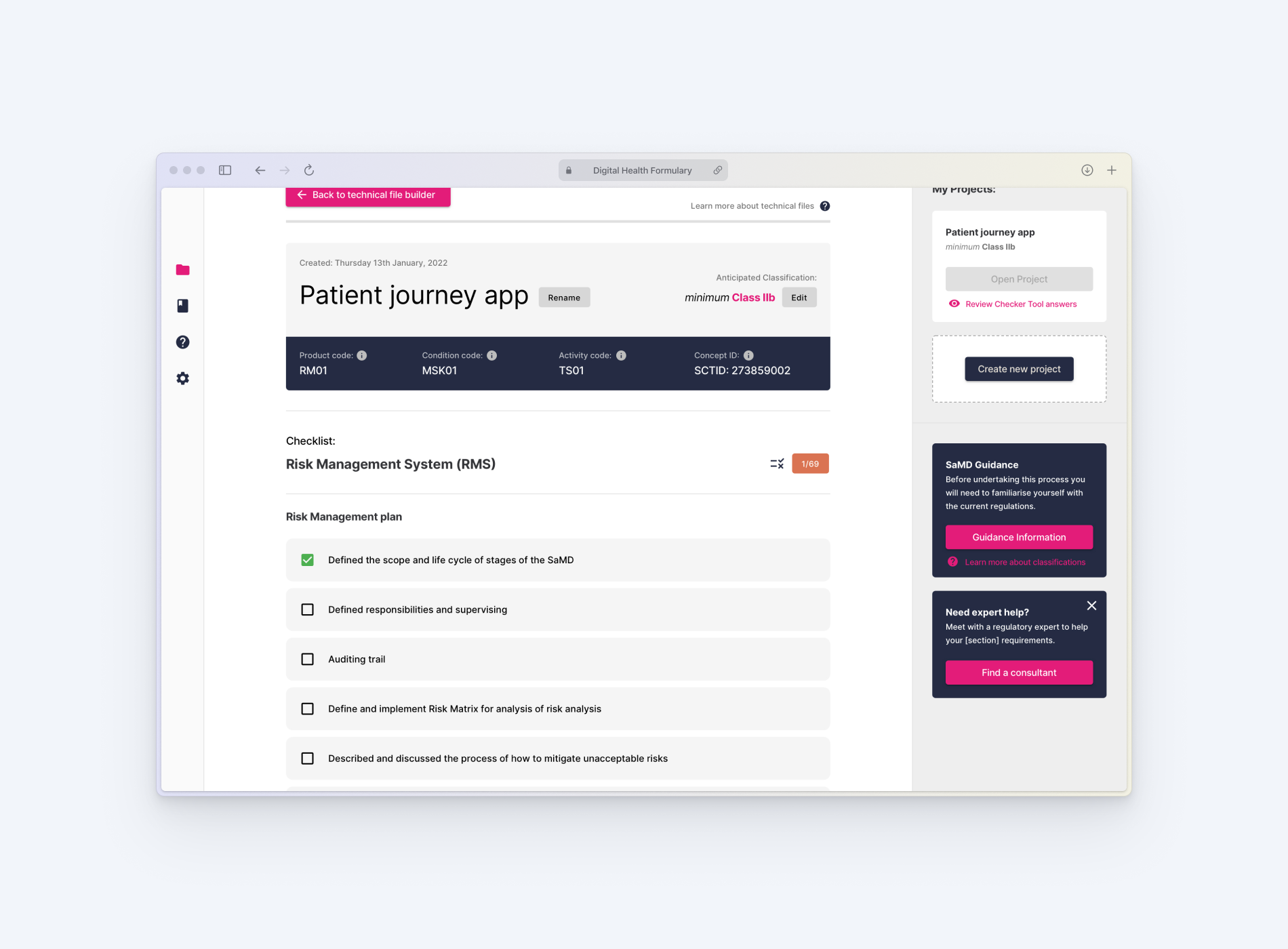
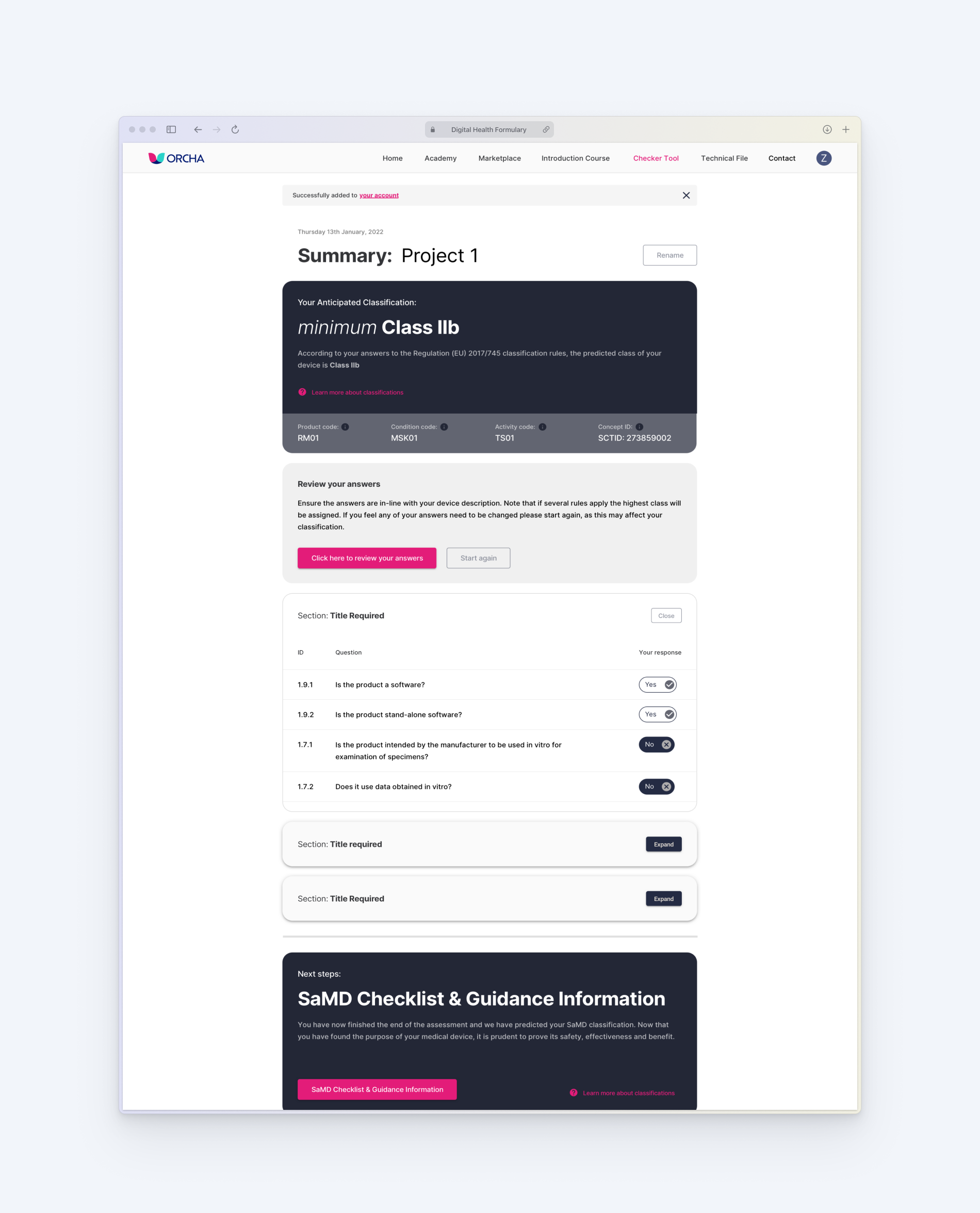
A logic-based tool that accurately determines whether a product qualifies as a medical device and predicts its classification under both UK and EU frameworks.
-
Generates a tailored list of requirements based on the product’s classification, written in plain English and structured around relevant domains like data privacy, clinical safety, and cybersecurity.
-
Guides users in collecting, organising, and exporting the documentation required for regulatory submissions — aligned to ISO and MDR standards.
-
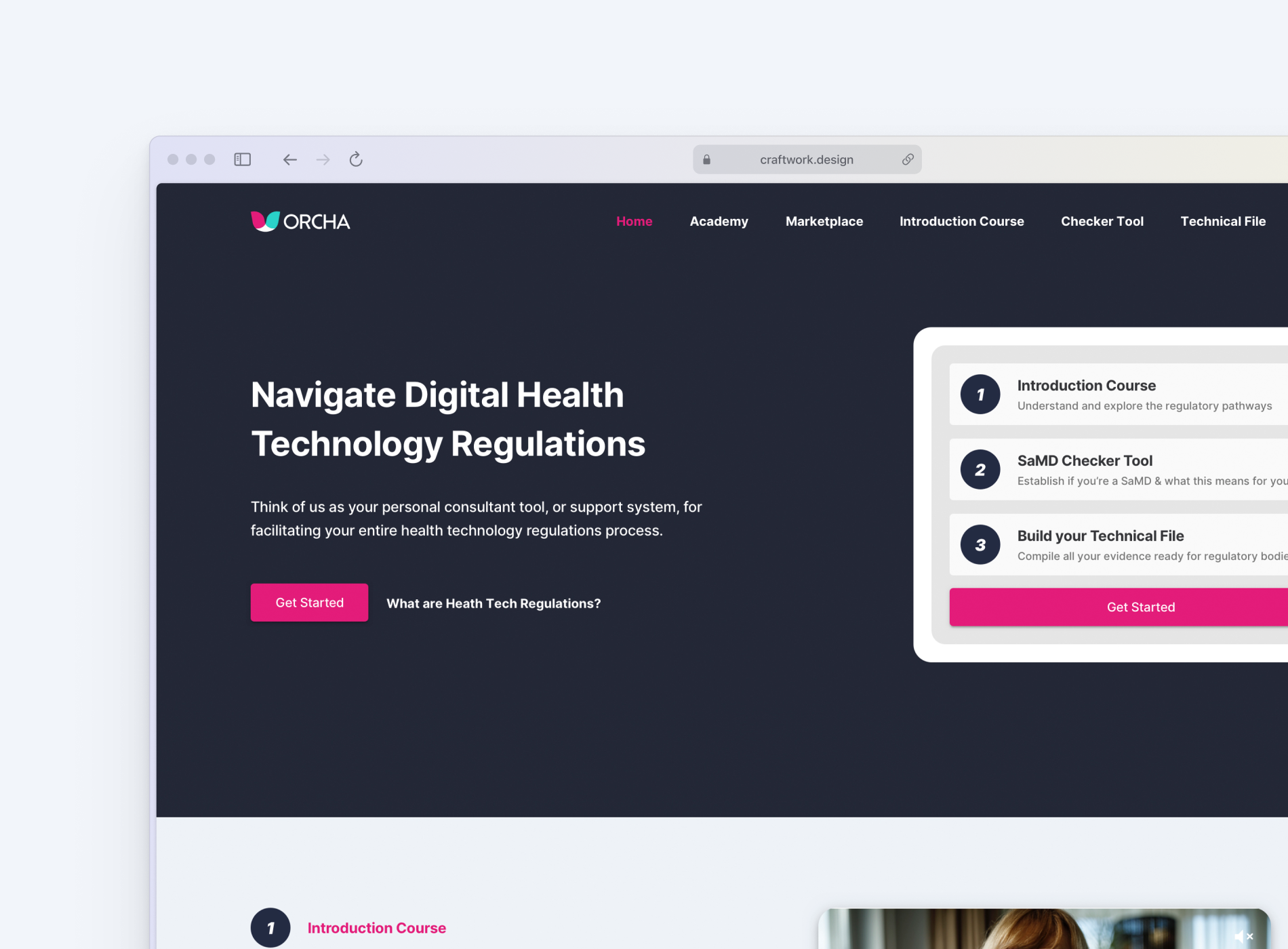
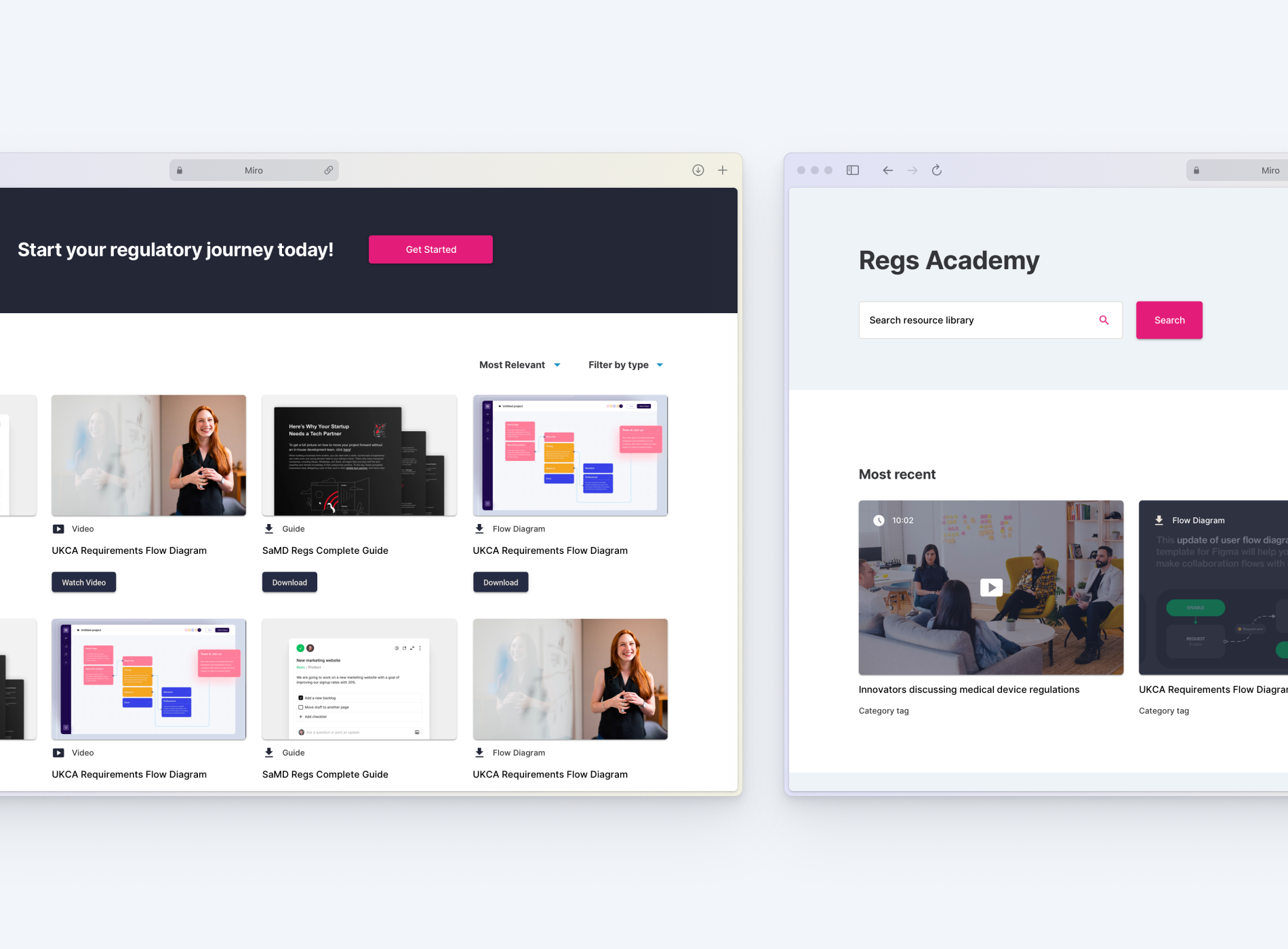
An integrated learning environment offering curated videos, explainers, and downloadable guides to demystify the regulatory process and empower independent learning.
-
Mapped digital health products against recognised clinical codes, identifying gaps and proposing standardised nomenclature use within the platform outputs.
-
Engaged with product owners, healthcare professionals, and regulatory stakeholders to test assumptions, refine prototypes, and validate real-world utility.
-
Captured both qualitative and quantitative feedback, confirming that the tool is perceived as time-saving, cost-effective, and valuable enough to pay for — while identifying areas for further refinement in Phase 2.
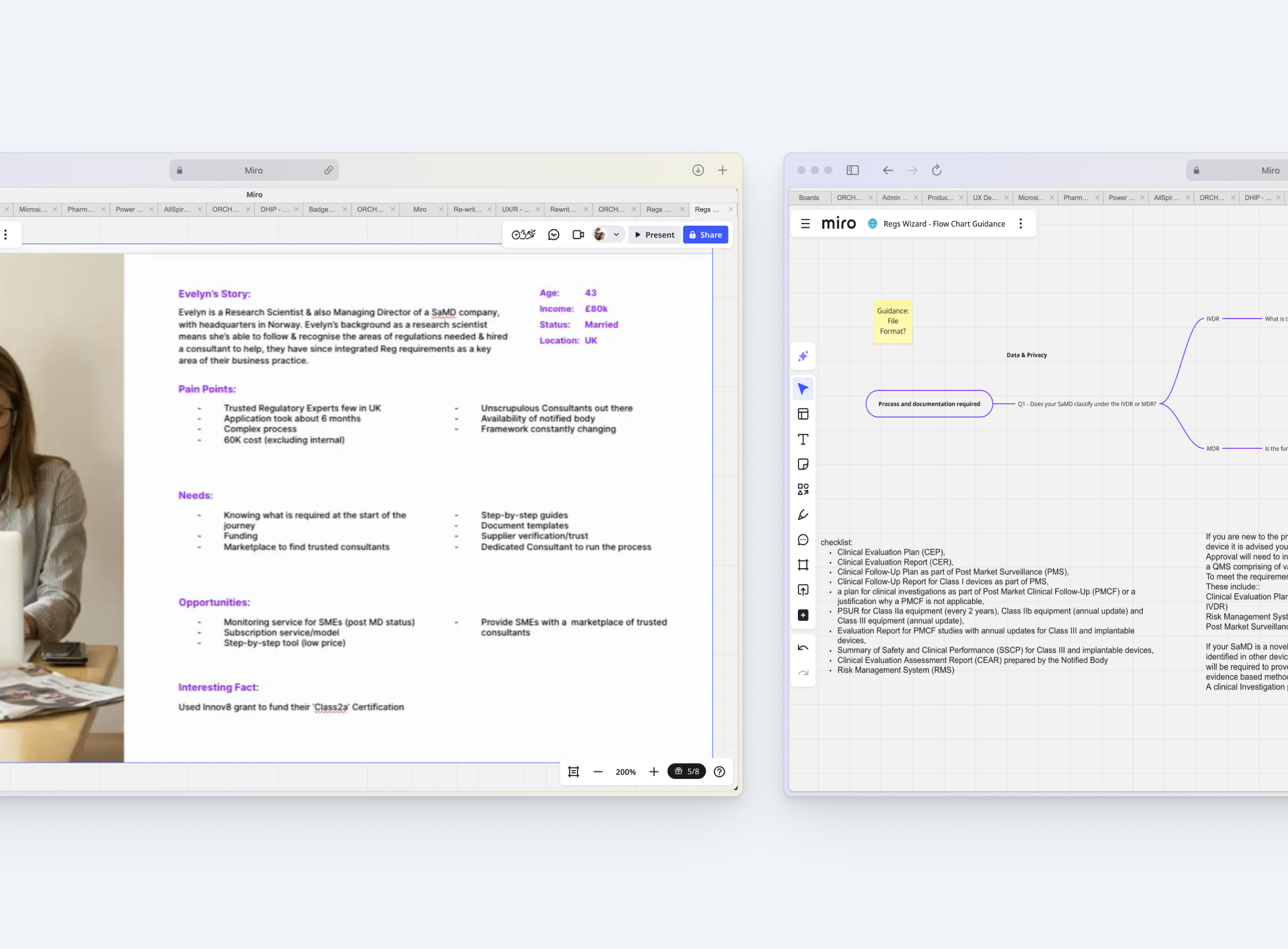
Above left: Example persona
Above right: Flow chart for decision checker tool
Process
Our approach combined user-centred design and collaborative action research.
This involved:
Conducting qualitative interviews with product owners and regulators
Mapping regulatory frameworks and decision logic into a usable UX
Prototyping in stages — tested and refined through multiple feedback loops
Designing a scalable pattern for future product categories (e.g. diagnostics, cosmetics)
The prototype included a logic-driven checker tool, modular technical file builder, and an integrated knowledge base — all backed by research and regulatory input.
Challenges
The medical device regulation landscape is inherently ambiguous and fragmented
Product owners lacked a consistent understanding of their obligations
Existing resources were either too generic or too costly
Mapping to standards (e.g. SNOMED, GMDN) required gap analysis and augmentation
Designing a product that worked for both experienced and novice users
Despite these, we delivered a fully functioning prototype with real-world validation.
Outcome
The final product received strong validation from users and stakeholders:
100% of participants said they would pay to use the tool
Reported benefits included time and cost savings, improved clarity, and reduced admin
The tool was described as a “one-stop shop” for SaMD regulatory readiness
Delivered a viable path to MVP and commercial launch in Phase 2
RegsWizard has laid the foundation for a scalable, intelligent regulatory platform — helping digital health innovators move faster, with confidence and compliance built in.
The prototype was tested by 32 product owners, including 22 healthcare professionals, using a structured feedback survey via UserTesting. The results validated both the concept and user experience, showing strong perceived value: Avg. Score (0–10)
While feedback on core value was overwhelmingly positive, scores also highlighted areas for UX improvement — specifically around guidance clarity and reducing admin friction. These insights will guide refinement in Phase 2.
Respondents consistently expressed willingness to pay for the tool, citing its potential to replace or complement costly consultancy support.
Above: Regs academy - Resources for understanding the SaMD regulations